The compound that i have chosen is Acetic Acid. Its symbol is CH3COOH. It is an organic acid that gives vinegar its sour taste and pungent smell. It is a weak acid, in that it is only a partially dissociated acid in an aqueous solution. Pure, water-free acetic acid (glacial acetic acid) is a colourless liquid that absorbs water from the environment (hygroscopy), and freezes at 16.5 °C (62 °F) to a colourless crystalline solid. The pure acid and its concentrated solutions are very corrosive.
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5730 years.
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of 1.00794 u, hydrogen is the lightest and most abundant chemical element, constituting roughly 75 % of the Universe's chemical elemental mass. It is highly flammable and ignites instantly when in contact with heat. It is colourless, odourless, nonmetallic and tasteless at room temperature.
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς (oxys) (acid, literally "sharp", referring to the sour taste of acids) and -γενής (-genēs) (producer, literally begetter), because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula O2.
Wednesday, February 9, 2011
Eric Seow (8)
Sulfur Trioxide is the chemical compound with the formula SO3. It is the primary agent of acid rain.
Formation
Sulfur Trioxide- 3 Oxygen atoms + 1 Sulfur atom
In terms of electron-counting formalisms, the three oxygen atoms are in the -2 oxidation state and the sulfur atom has an oxidation state of +6, a formal charge of 0, and is surrounded by 6 electron pairs.
Constituent Elements
The most important use of oxygen is for breathing. Animals require oxygen in their air (or water) to live.
Another use for oxygen is for oxidation reactions -- the most obvious
example is burning. Fires require oxygen to burn. Bonding Sulfur trioxide is formed from a covalent bond. Dot and cross diagram Sun Yu Hsiang (25)
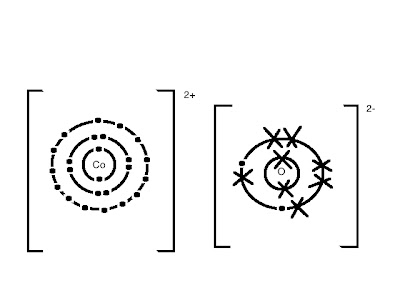
The harmful compound that I chose is Cobalt Oxide. It is made up of 1 cobalt atom (Co) and 1 oxygen atom (O). Its atomic symbol is CoO. These 2 elements bond using ionic bonding. Cobalt is a harmless metal, while oxygen is a harmless gas. However, Cobalt Oxide is dangerous to humans and harmful to the environment.
Dot-and-Cross Diagram:
Dot-and-Cross Diagram:
Declan Lee
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula NO2. One of several nitrogen oxides, NO2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant. Nitrogen dioxide is a paramagnetic bent molecule with C2v point group symmetry.
covalent bond
covalent bond
Joseph Tan :)
Hydrogen Bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation.
At room temperature, HBr is a nonflammable gas with an acrid odor, fuming in moist air because of the formation of hydrobromic acid. HBr is very soluble in water, forming hydrobromic acid solution, which is saturated at 68.85%. Aqueous solutions that are 47.38% HBr by weight form a constant-boiling mixture that boils at 126°C.
There are many uses of HBr in chemical synthesis. For example, HBr is used for the production of alkyl bromides from alcohols.
Hydrogen is an element with an atomic number of 1. It is the lightest and most abundant chemical element. Bromine has an atomic number of 35 and an atomic mass of 79.904.



Chairul Karim (2)
Hydrogen Peroxide(H2O2)
Covalent Bonding
It is made up of 2 hydrogen atoms and 2 oxygen atoms. The 2 hydrogen atoms share one electron and then the 2 hydrogen atoms share an electron each to each side of the oxygen atoms, thus forming the compound.

Covalent Bonding
It is made up of 2 hydrogen atoms and 2 oxygen atoms. The 2 hydrogen atoms share one electron and then the 2 hydrogen atoms share an electron each to each side of the oxygen atoms, thus forming the compound.

Nigel Tan's Post (22)
Octyl methodxycinnamate. Used in sunscreen and eventually sinks into the deeper layers of the skin and makes the top layer vulnerable.
Koh Kai Jie
Carbonic Acid
Carbonic Acid is made up of Hydrogen(H), Carbon(C) and Oxygen(O2)
Carbonic Acid is formed naturally in our blood and is the indicator the body uses when it lacks air. this will cause fainting and drowning.
Carbonic Acid is made up of Hydrogen(H), Carbon(C) and Oxygen(O2)
Carbonic Acid is formed naturally in our blood and is the indicator the body uses when it lacks air. this will cause fainting and drowning.
Kee Hsien's post- Sodium Bromide
Sodium bromide- made of Sodium(Na) and bromine(Br)
Sodium bromide (NaBr).
Composed of Sodium (Na) which is from the 3rd element of Group 1. It is the largest atom of its period, however still relatively small.
1st Ionization Energy: 495.8 kJ·mol−1
Electronegativity: 0.93 (Pauling scale)
and Bromine (Br) with is the 3rd element of Group 17. It is a small atom, given its position on the right side of the periodic table.
1st Ionization Energy: 1139.9 kJ·mol−1
Electronegativity: 2.96 (Pauling scale)
Composed of Sodium (Na) which is from the 3rd element of Group 1. It is the largest atom of its period, however still relatively small.
1st Ionization Energy: 495.8 kJ·mol−1
Electronegativity: 0.93 (Pauling scale)
and Bromine (Br) with is the 3rd element of Group 17. It is a small atom, given its position on the right side of the periodic table.
1st Ionization Energy: 1139.9 kJ·mol−1
Electronegativity: 2.96 (Pauling scale)
Sodium bromide (NaBr) is a single salt clear brine fluid that has the following features and benefits:
- It is non-damaging to the formation,
- It is thermally and chemically stable,
- It can be blended with other solutions of bromides and chlorides, and
- It is especially useful when used in formations that are known to have calcium sensitivity.
A pure sodium bromide (NaBr) brine fluid is often selected when the chloride ion is not desirable and when sodium is preferred over calcium. It is often used in situations where formation waters contain high levels of sulfate or carbonate that may precipitate with the calcium ion.
Goh Zhi Hwee (09)- Benzene
Benzene (C6H6)
An organic chemical compound, Benzene is a colourless and flammable liquid with a sweet smell. It is used as an industrial solvent in the production of products such as drugs, plastics and synthetic rubber, but after it was discovered to be a known carcinogen, its use in the manufacturing of these products has been limited. It has a molacular mass of 78.11g mol and a continuous pi bond.
Today, benzene is used mainly as an intermediate to make other chemicals. If exposed to benzene, it can lead to serious health problems like leukemia. It damages the bone marrow and can also decrease the number of red blood cells. When ingested, benzene causes vomiting, dizziness, convulsions and eventually death.
In laboratory research, toluene is now used as a substitute for benzene as it is less toxic and has a wider liquid range.
About the elements, carbon is an element which has an atomic number of 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent. It can exist in three different forms, such as diamond, graphite and soot. Hydrogen is a chemical element with an atomic number of 1. It has an atomic weight of 1.00794 units.
About the elements, carbon is an element which has an atomic number of 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent. It can exist in three different forms, such as diamond, graphite and soot. Hydrogen is a chemical element with an atomic number of 1. It has an atomic weight of 1.00794 units.
 |
| Atomic structure of Benzene. |
Soh Hwa Yang 24
beryllium fluoride
Beryllium fluoride is the inorganic compound with the formula BeF2.Gaseous BeF2 is found in the gas-phase above 1160 °C.Beryllium fluoride is used in biochemistry, particularly protein crystallography as a mimic of phosphate.All beryllium compounds are highly toxic. Beryllium fluoride is very soluble in water and is thus absorbed easily.
dot and cross diagram
Beryllium fluoride is the inorganic compound with the formula BeF2.Gaseous BeF2 is found in the gas-phase above 1160 °C.Beryllium fluoride is used in biochemistry, particularly protein crystallography as a mimic of phosphate.All beryllium compounds are highly toxic. Beryllium fluoride is very soluble in water and is thus absorbed easily.
dot and cross diagram
Yap Jun Lin
Sodium Hydroxide
Sodium Hydroxide (NaOH) in solution is very strongly alkaline or basic. At even low concentrations it is dangerously corrosive to skin and eyes. The bond that binds the hydrogen (H) to the oxygen (O) is covalent. The sodium (Na) is bonded to the hydroxide part of the compound with an ionic bond.
Hydrogen is the chemical element with the atomic number 1. It is represented by the symbol H. Hydrogen is the lightest and most abundant chemical element, constituting 75% of the Universe's chemical elemental mass. Sodium is a metallic element with a symbol Na and atomic number 11. It is a soft, silvery-white metal and is a member of the alkali metals within group 1. It has only one stable isotope. Chemical equation for the formation of sodium hydroxide:2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g).
Sodium Hydroxide (NaOH) in solution is very strongly alkaline or basic. At even low concentrations it is dangerously corrosive to skin and eyes. The bond that binds the hydrogen (H) to the oxygen (O) is covalent. The sodium (Na) is bonded to the hydroxide part of the compound with an ionic bond.
Hydrogen is the chemical element with the atomic number 1. It is represented by the symbol H. Hydrogen is the lightest and most abundant chemical element, constituting 75% of the Universe's chemical elemental mass. Sodium is a metallic element with a symbol Na and atomic number 11. It is a soft, silvery-white metal and is a member of the alkali metals within group 1. It has only one stable isotope. Chemical equation for the formation of sodium hydroxide:2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g).
Wan Wei Ren (31)
Nitric Oxide
Nitric OxideàNitrogen + Oxygen (NO)
Nitric Oxide is a polar covalent bond. Nitric Oxide is a covalent bond because it is made up of 1 atom of Nitrogen and Oxygen each and since Nitrogen has 5 valence electrons and Oxygen has 6 valance electrons, they form a covalent bond by sharing electrons. Nitric Oxide is a polar bond because electrons are unequally shared.
Nitric Oxide is made up of Nitrogen and Oxygen. Nitrogen (N) has an atomic number of 7 and atomic mass of 14.00674 u. It is a colorless, odorless and tasteless gas. Nitrogen is generally unreactive at standard temperature and pressure and it is used to preserve packaged food, used to produce many electrical parts such as transistors, diodes and integrated circuits, and used to make stainless steel. Oxygen (O) has an atomic number of 8. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula for O2. Oxygen is used for combustion and welding and melting. Nitric oxide is formed from nitrogen and oxygen by the action of electric sparks or high temperatures or, more conveniently, by the action of dilute nitric acid upon copper or mercury.
Dot and cross diagram below
Bryan Chia Jun Qing (5)
Carbon + Sulfur => Carbon disulfide
Carbon disulfide is formed from covalent bonding between carbon and sulphur. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent. It has an "ether-like" odour, but commercial samples are typically contaminated with foul-smelling impurities, such as carbonyl sulfide.
Carbon is the chemical element with symbol C and atomic number 6. It is non-metallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5730 years. Carbon is one of the few elements known since antiquity.
Sulfur is the chemical element that has the atomic number 16. It is denoted with the symbol S. It is an abundant, multivalent non-metal. Sulfur, in its native form, is a bright yellow crystalline solid. In nature, it can be found as the pure element and as sulfide and sulfate minerals. Its commercial uses are primarily in fertilizers, but it is also widely used in black gunpowder, matches, insecticides and fungicides. Elemental sulfur crystals are commonly sought after by mineral collectors for their brightly coloured polyhedron shapes.
Soh Han Qiang (23)
Hydrogen Peroxide—formed by two atoms of Hydrogen and Oxygen each
These two elements are bonded together by covalent bonding.
Hydrogen is a constituent element of hydrogen peroxide with the symbol H. It is the most common element in the universe, found in almost every star. It is highly flammable and ignites instantly when in contact with heat. It is colourless, odourless, nonmetallic and tasteless at room temperature.
Oxygen is a constituent element of hydrogen peroxide with the symbol O. By mass, it is the third most abundant element in the universe after hydrogen and helium. It is required by all living things for respiration. At room temperature, it is a colourless odourless and tasteless gas.
Formation
As oxygen has 8 electrons and 6 valence electrons, 2 more valence electrons are needed to obtain a full valence shell. A hydrogen atom has 1 valence electron. Since Hydrogen Peroxide has two hydrogen atoms, they share a single bond of electrons. Since they lack one electron, the hydrogen atom will share its valence electron to form a full valence shell.
Tan Zi Jie(30)2P1
Chromium oxide
Heating with chlorine and carbon yields chromium(III) chloride:
 Chromium oxide can be converted into elemental chromium metal through a thermite-like reaction: unlike iron oxide thermites, chromium oxide thermites creates few or no sparks, smoke or sound, but glow brightly. Because of the very high melting point of chromium, chromium thermite casting is impractical.
Chromium oxide can be converted into elemental chromium metal through a thermite-like reaction: unlike iron oxide thermites, chromium oxide thermites creates few or no sparks, smoke or sound, but glow brightly. Because of the very high melting point of chromium, chromium thermite casting is impractical.
Heating with chlorine and carbon yields chromium(III) chloride:
- Cr2O3 + 3 Cl2 + 3 C → 2 CrCl3 + 3 CO
 Chromium oxide can be converted into elemental chromium metal through a thermite-like reaction: unlike iron oxide thermites, chromium oxide thermites creates few or no sparks, smoke or sound, but glow brightly. Because of the very high melting point of chromium, chromium thermite casting is impractical.
Chromium oxide can be converted into elemental chromium metal through a thermite-like reaction: unlike iron oxide thermites, chromium oxide thermites creates few or no sparks, smoke or sound, but glow brightly. Because of the very high melting point of chromium, chromium thermite casting is impractical.
Iven Lim (17)
Hydrogen cyanide (HCN) is a harmful compound formed by hydrogen, carbon and nitrogen of one atom each. The type of chemical bonding involved is covalent bonding. Hydrogen (H) has only 1 electron. It is the fuel that powers the Sun and other stars. Carbon (C) has 6 electrons. All life on earth is made from it. It has three allotropes which are graphite, diamond and amorphous carbon. Nitrogen (N) has 7 electrons. It makes up about 78% of the air and is used to prevent things from reacting with the oxygen in the air. When the three elements are put together, they react so that hydrogen forms a bond with carbon and carbon forms three bonds with nitrogen. Hence the valence shells of all the constituent elements will be fully-filled and hydrogen cyanide will be formed.
Chen Zhaofeng (04)
Magnesium Chloride (MgCl 2) - One atom of Magnesium + Two atoms of Chlorine
Magnesium Chloride is formed through ionic bonding of Magnesium and Chlorine. Magnesium has 2 valence electrons while Chlorine has 6 valence electrons. In order for the formation Magnesium Chloride, Magnesium must give away his 2 valence electrons to Chlorine while Chlorine must receive 1 more electron from Magnesium to form a fully-filled valence shell. In order for Magnesium to give away its 2 valence electron, two atoms of chlorine needed as an atom of Chlorine only requires one electron. Thus Magnesium Chloride is formed when 1 atom of Magnesium combines with 2 atoms of Chlorine.
Dot and Cross Diagram
Cheh Fu Yang (03)
Calcium Chloride (CaCl_2).
This compound is formed by using ionic bonding to bond Calcium ions(Ca) and 2 Chlorine ions (Cl). The compound is formed when Calcium ions give 1 electrons each to the two chloride ions for both of them to be stable.
Calcium also strenghten bones in your body
Chlorine
Chlorine can be used in disinfection and purification and plays an important part of water purification.
Chlorine is used on the making of plastics and other consumer products
Chlorine is used in the production of chlorates and beomine extraction
Calcium Chloride
If Calcium Chloride is ingested it will burn the mouth and gullet.
This compound is formed by using ionic bonding to bond Calcium ions(Ca) and 2 Chlorine ions (Cl). The compound is formed when Calcium ions give 1 electrons each to the two chloride ions for both of them to be stable.
Calcium
Calcium is used as a deoxidizer, desulfurizer, or decarbonizer for various ferrous and nonferrous alloys.
Calcium is used in the making of cheese, where calcium ions influence the activity of rennin in bringing about the coagulation of milk.Calcium also strenghten bones in your body
Chlorine
Chlorine can be used in disinfection and purification and plays an important part of water purification.
Chlorine is used on the making of plastics and other consumer products
Chlorine is used in the production of chlorates and beomine extraction
Calcium Chloride
If Calcium Chloride is ingested it will burn the mouth and gullet.
Nesman Ng (21)
The harmful compound is Methane (CH4), formed by one atom of Carbon and four atoms of Hydrogen. Methane is formed by a covalent bond. Hydrogen has one valence electron while Carbon has four valence electron. For a Hydrogen atom to be stable, it needs one more valence electron. Carbon needs four more valence electrons to become stable. As Methane consists of four Hydrogen atoms and one Carbon atom, a covalent bond is formed.
Carbon is the chemical element with symbol C and atomic 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of 1.0079, hydrogen is the lightest and most abundant chemical element, constituting roughly 75 % of the Universe's chemical elemental mass. Methane is a relatively potent greenhouse gas. Compared with carbon dioxide, it has a high global warming potential of 72 (more harmful than CO2 as a greenhouse gas). Methane is not toxic; however, it is highly flammable and may form explosive mixtures with air. Methane is also an asphyxiant and may displace oxygen in an enclosed space. Asphyxia may result if the oxygen concentration is reduced to below 19.5% by displacement.
Brian Low 18
The compound that i have chosen is cyanide.The cyanide anion (CN-) is an example of an ionic species characterized by covalent bond. It is composed of one carbon atom and one nitrogen atom bound by a triple bond. An unshared electron pair is present on both the carbon and the nitrogen atoms It is bonded by covalent bonding, by a triple bond with means that each atom shares 3 electrons. Cyanide is formed by carbon and nitrogen. Carbon and Nitrogen by itself are non-harmful elements.
Carbon atoms have 6electrons so its electronic configuration is 2.4 and Nitrogen atoms have 7 electrons so its electronic configuration is 2.5. As they are both non-metals, they combine through covalent bonding. However, when they form a compound,Cyanide,it will have a negative charge. It is because the there is one more delocalized electron so it cannot form a stable structure it is toxic and lethal to the human beings as it absorbs the oxygen in the bloodstream and cause death.
Carbon atoms have 6electrons so its electronic configuration is 2.4 and Nitrogen atoms have 7 electrons so its electronic configuration is 2.5. As they are both non-metals, they combine through covalent bonding. However, when they form a compound,Cyanide,it will have a negative charge. It is because the there is one more delocalized electron so it cannot form a stable structure it is toxic and lethal to the human beings as it absorbs the oxygen in the bloodstream and cause death.
Wesley Chai
The harmful compound that i have chosen is ethanoic acid(CH3COOH)
Ethanoic acid is made up of carbon (C), hydrogen (H), and oxygen (O).
Carbon
Oxygen
Ethanoic acid is made up of carbon (C), hydrogen (H), and oxygen (O).
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is non-metallic and tetravalent—making four electrons available to form covalent chemical bonds.
Hydrogen
Hydrogen is the simplest element of all, and the lightest. It is also by far the most common element in the Universe. Over 90% of the atoms in the Universe are hydrogen. In its commonest form, the hydrogen atom is made of one proton, one electron, and no neutrons. Hydrogen is the only element that can exist without neutrons. Hydrogen is a colourless, odourless gas which exists, at standard temperature and pressure, as diatomic molecules, H2. It burns and forms explosive mixtures in air and it reacts violently with oxidants.
Oxygen, in its natural state, is a colourless, odourless, and tasteless gas. Oxygen is considered to be the most important of all the elements tolife. It forms about 21% of the atmosphere by volume and 23% by weight. Of all the elements in our environment, oxygen is the most plentiful. It makes up nearly one-half of the earth’s crust and approximately one-fifth of the air we breathe.

Stephen Sun (26)
The harmful compound I have chosen is sulfuric acid (H2SO4 )( historically known as vitriol). Its constituent elements are hydrogen, oxygen and sulfur. It is formed by covalent bonding.
Hydrogen is a constituent element of sulfuric acid with the symbol H. It is the most common element in the universe, found in almost every star. It is highly flammable and ignites instantly when in contact with heat. It is colourless, odourless, nonmetallic and tasteless at room temperature.
Oxygen is a constituent element of sulfuric acid with the symbol O. By mass, it is the third most abundant element in the universe after hydrogen and helium. It is required by all living things for respiration. At room temperature, it is a colourless odourless and tasteless gas.
Sulfur is a constituent element of sulphuric acid with the symbol S. It is an abundant, multivalent non-metal. Sulfur, in its native form, is a bright yellow crystalline solidIt is an essential element for life and is found in two amino acids: cysteine and methionine.
When the three elements above are combined, it will form sufuric acid. Sufuric acid is highly corrosive as it reacts with water easily. Burns from sulfuric acid are potentially more serious than those of other acids as there is additional tissue damage due to dehydration and secondary heat damage from the heat produced when reacting with water. Sulfuric acid has many applications, and is a central substance in the chemical industry. Principal uses include lead-acid batteries for cars and other vehicles, ore processing, fertilizer manufacturing, oil refining, wastewater processing, and chemical synthesis.
Wesley Kam (12)
Formaldehyde (Methylene Oxide)
Formaldehyde is an organic compound with the formula CH2O. It is made up of 1 atom of carbon, 2 atoms of hydrogen and 1 atom of oxygen.
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is non-metallic and tetravalent—making four electrons available to form covalent chemical bonds.
Hydrogen is the simplest element of all, and the lightest. It is also by far the most common element in the Universe. Over 90% of the atoms in the Universe are hydrogen. In its commonest form, the hydrogen atom is made of one proton, one electron, and no neutrons. Hydrogen is the only element that can exist without neutrons. Hydrogen is a colourless, odourless gas which exists, at standard temperature and pressure, as diatomic molecules, H2. It burns and forms explosive mixtures in air and it reacts violently with oxidants.
Oxygen, in its natural state, is a colourless, odourless, and tasteless gas. Oxygen is considered to be the most important of all the elements to life. It forms about 21% of the atmosphere by volume and 23% by weight. Of all the elements in our environment, oxygen is the most plentiful. It makes up nearly one-half of the earth’s crust and approximately one-fifth of the air we breathe.
Carbon has 4 valence electrons, hydrogen has 1 valence electron, while oxygen has 6 valence electrons. All the constituent elements are also non-metallic Thus, I can conclude that covalent bonding is involved.
Subscribe to:
Comments (Atom)